"The conclusion from
these arguments presents the most serious obstacle, if indeed it is not fatal, to the
theory of spontaneous generation. First, thermodynamic calculations predict vanishingly
small concentrations of even the simplest organic compounds. Secondly, the reactions that
are invoked to synthesize such compounds are seen to be much more effective in decomposing
them."—*D. Hull, "Thermodynamics and Kinetics of Spontaneous Generation,
" in Nature, 186 (1960), pp. 693—694.
"In other words, the theoretical
chances of getting through even this first and relatively easy stage [getting amino acids]
in the evolution of life are forbidding."—*Francis Hitching, The Neck
of the
Giraffe (1982), p. 65.
But there is still more:
In contrast, "Biogenesis"
is the scientific name for the important biological truth confirmed by Louis Pasteur
and others, that life can only come from life.
"Biogenesis is a term
in biology that is derived from two Greek words meaning life and birth. According
to the theory of biogenesis, living things descend only from living things. They cannot
develop spontaneously from nonliving materials. Until comparatively recent times,
scientists believed that certain tiny forms of life, such as bacteria, arose spontaneously
from non—living substances."— * "Biogenesis, " in World Book
Encyclopedia, p. B—242. (1972 edition.)
Spontaneous generation was believed by
many scientists prior to the careful experiments of Spallanzani (1780), and Pasteur
(1860), which totally disproved that foolish idea. People thought that fruit flies
spontaneously came forth from fruit, geese from barnacles, mice from dirty clothes, and
bees from dead calves. Even Copernicus, Galileo, Bacon, *Hegel, and *Shilling believed
it, but that did not make it right. Great people believing an error does not make the
error truth.
Evolution teaches spontaneous
generation. Think about that for a moment. We're returning to the Dark Ages!
"Pasteur's
demonstration apparently laid the theory of spontaneous generation to rest permanently.
"All this left a germ of
embarrassment for scientists. How had life originated after all, if not through divine
creation or through spontaneous generation? . . this
left a germ of embarrassment for scientists. How had life originated after all, if not
through divine creation or through spontaneous generation? . .
"They [scientists] are [today] back
to spontaneous generation, but with a difference. The pre-Pasteur view of spontaneous
generation was of something taking place now and quickly. The modern view is
that it took place long ago and very slowly."—*Isaac Asimov, Asimov's New
Guide to Science (1984), pp. 638—639.
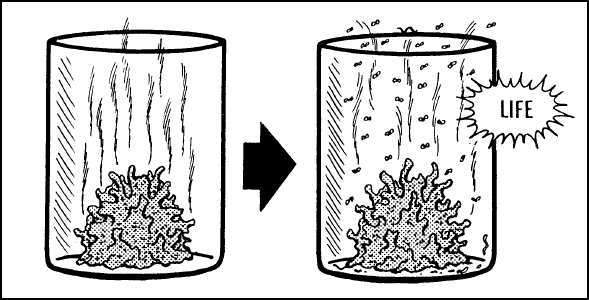
LIFE COMES ONLY FROM LIFE—Vegetation
is placed in an open container. Seeds within the vegetation sprout. Living creatures have
access to it, so they come, feed, and reproduce their young. Life continues on, but it did
not originate within this jar.
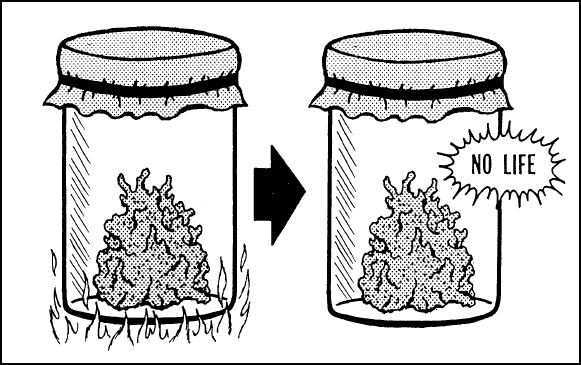
NON-LIFE CANNOT PRODUCE
LIFE—Food
is placed in a closed container and then heated sufficiently to kill all living organisms
and their seeds. It is kept closed and no life originates within it or within the
atmosphere surrounding it. Life will not originate within this jar, and if it appears to,
the cause will have been insufficient initial heat to kill all life spores (mold, etc.)
brought in from outside.
In contrast, true science teaches
"biogenesis," which means, in general, that life can only come from life,
and, specifically, that species can only come from living parents in the same species.
Speaking of Rudolf Virchow, the Encyclopedia Britannica tells us: and, specifically, that species can only come from living parents
in the same species. Speaking of Rudolf Virchow, the Encyclopedia Britannica tells
us:
"His aphorism 'omnis
cellula a cellula' [every cell arises from a pre-existing cell] ranks with Pasteur's
`omne
vivum a vivo' [every living thing arises from a pre-existing living thing] as among
the most revolutionary generalizations of biology."—*Encyclopedia Britannica,
1973 Edition, Volume 23, p. 35.
" 'Every cell from a
cell.'—Rudolf Virchow, German pathologist 'Every living thing from a living
thing.' 'Spontaneous generation is a chimera [illusion].'—Louis Pasteur, French
chemist and microbiologist."—Quotations in Isaac Asimov's Book of Science and
Nature Quotations (1988), p. 193.
In the year 1860, Louis Pasteur, a
creationist, concluded experiments a year after the publication of *Darwin's Origin of the
Species, showing that broth in sterile flasks did not spoil. Microbes from outside could
not get in, and germs could not generate spontaneously inside the flasks although both
broth and air inside were ready to support them. His work demolished the ancient idea,
held at least since the time of the Greeks, that flies spontaneously create themselves out
of manure, fruit flies from fruit, and frogs from pond water.
Pasteur's concept, called the law of biogenesis,
holds that life only comes from living material of the same kind. This law is taught
in every basic biology class in our schools. But, down the hall in the historical biology
class, the students are taught a totally opposite "principle," a fundamental
error of evolution: living things originated from non-living materials.. This law is
taught in every basic biology class in our schools. But, down the hall in the historical
biology class, the students are taught a totally opposite "principle," a
fundamental error of evolution: living things originated from non-living materials.
For additional
information see quotation supplement, "2 — Spontaneous Generation," at the
end of this chapter.
INSTANT SUCCESS NECESSARY—In order
for life to arise from non-life, there would have to be instant success. All the parts
would suddenly have to be there, and all would have to function with essential perfection.
In the next chapter (chapter 10), we will
learn that, in order for life to occur, DNA a protein would have to link up with ease into
long, extremely complicated coded strings. In addition, thousands of other complicated
chemical combinations would have to be accomplished within a few moments. How long could
you live without a beating heart? how long without blood? And on it goes, item after item.
The situation would be no different for the simplest of life forms. Everything would have
to be in place—suddenly—instantly. In structure, arrangement, coordination,
coding, chemical makeup, feeding, elimination, respiration, circulation, and all the
rest,—everything would have to be perfect!
IMMEDIATE REPRODUCTION
NEEDED—Biologists are deeply concerned how that first living cell could have
originated, but *Montalenti goes a step beyond that point and says "what really
matters, to start life is . . the faculty of reproduction." (*G. Montalenti,
Studies in the Philosophy of Biology (1974), p. 13.) What good would one cell be if
it did not have all the needed DNA coding and fission ability to divide, or the
reproduction ability—and a mate—to produce offspring?
3 — CHEMICAL COMPOUNDS
CHEMICAL COMPOUNDS AND LABORATORIES—Complicated
chemical compounds are prepared in well-equipped laboratories, staffed by intelligent,
highly skilled workers. They do not work with the sand in the back lot, but with shipments
of specialized chemicals which arrive at their loading dock.
About all that most evolutionists offer
for the original primitive environment for the first amino acids, proteins, etc., is dirt
or sea water. Yet when scientists want to synthesize amino acids, they go to a very
well-equipped laboratory, with instruments, gauges, apparatus, chemicals, and machines
costing hundreds of thousands of dollars. They use high-temperatures, special solutions,
sparking devices, and glass traps. They do not go down to the sea shore and start sloshing
around in sea water in the hope of producing those amino acids.
Because they are intelligent and trained,
they know to do it in laboratories fitted out with expensive equipment and jars of
chemicals; yet, according to evolutionary theory, sea water somehow did it by itself.
CHEMICAL COMPOUNDS AND THE LAW OF MASS
ACTION—Evolutionists recognize that, if a life form suddenly appeared from
nothing, it would probably have had to do it in an ancient sea. It is generally felt that
water would have had to be present.
But "the Law of Mass Action"
would immediately neutralize the procedure and ruin the outcome. This is because chemical
reactions always proceed In a direction from highest to lowest concentration (assuming
that the exact amount of energy is even present to perform that reaction). (assuming that the exact amount of energy is even present to
perform that reaction).
"It is therefore hand to see how
polymerization [linking together smaller molecules to form bigger ones] could have
proceeded in the aqueous environment of the primitive ocean, since the presence of water
favors depolymerization [breaking up big molecules into simpler ones] rather than
polymerization."—*Richard E. Dickerson, "Chemical Evolution and the Origin
of Life, "Scientific American, September 1978, p. 75.
We are told that amino acids miraculously
formed themselves out of sea water. But the sea water, needed to make the amino acids,
would prevent them from forming into protein, lipids, nucleic acids and polysaccharides! Even
if some protein could possibly form, the law of mass action would immediately become
operative upon it. The protein would hydrolyze with the abundant water and return back
into the original amino acids! Those, in turn, would immediately break down into separate
chemicals—and that would be the end of it.
"Spontaneous dissolution is much more
probable, and hence proceeds much more rapidly, than spontaneous synthesis . . [This fact
is] the most stubborn problem that confronts us." —*George Wald, "The
Origin of Life," Scientific American, August 1954, pp. 49-50.
The law of mass action would constitute a
hindrance to protein formation in the sea as well as to the successful formation of other
life-sustaining compounds, such as lipids, nucleic acids, and poly-saccharides. If any
could possibly form in water, they would not last long enough to do anything.
This law applies to chemical reactions
which are reversible,—and thus to all life compounds. Such reactions proceed from
reactant substances to compounds produced in the manner normally expected. But these
reactions tend to reverse themselves more easily and quickly.
" 'All molecules
result from an electrochemical tendency to neutralization. They are therefore expressions
of tendencies toward stability.' Unhappily for materialists, however, life is
characteristically unstable, and 'it is incredible that the complex of substances, all
tending towards a state of stability, would produce the permanent chemical instability
which is characteristic of animate matter.' Thus it is inconceivable that an organic
compound should ever be formed in the absence of life: 'No condition of inorganic matter
is even thinkable in which carbon, oxygen and hydrogen could combine to form a sugar
rather than water and carbon dioxide."—*"Review of R. Shubert-Soldem's
Book, Mechanism and Vitalism," in Discovery, May 1962, p. 44.
Not just a few, but hundreds of thousands
of amino acids had to miraculously make themselves out of raw sea water devoid of any
life. But the amino acids would separate and break up immediately and not remain in
existence long enough to figure out how to form themselves into the complex patterns of
DNA and protein. The problem here is that, as soon as the chemical reaction occurred,
that made the amino acids, the excess water would have had to be removed immediately.
"Dehydration
[condensation] reactions are thermodynamically forbidden in the presence of excess
water."—*J. Keosian, The Origin of Life, p. 74.
CHEMICAL COMPOUNDS AND CONCENTRATION—Evolutionists
generally recognize that only warm sea water, by the edge of some ancient sea, could
provide the needed environment (although admittedly a very poor one) for amino acids to
appear. But we never find the concentrations of chemicals in sea water that would be
needed for amino acid synthesis. All the elements are there, but not in the proper
concentrations. Most of what is in sea water—is just water!
"it is commonly
assumed today that life arose in the oceans. . But even if this soup contained a goodly
concentration of amino acids, the chances of their forming spontaneously into long chains
would seem remote. Other things being equal, a dilute hot soup would seem a most unlikely
place for the first polypeptides to appear. The chances of forming tripeptides would be
about one-hundredth that of forming dipeptides, and the probability of forming a
polypeptide of only ten amino acid units would be something like 1/1020. The
spontaneous formation of a polypeptide of the size of the smallest known proteins seems
beyond all [mathematical] probability."—*H.F. Blum, Time's Arrow and
Evolution (1968), p. 158.
For additional
information see quotation supplement, "3 - The Primitive Ocean," at the end of
this chapter.
CHEMICAL COMPOUNDS AND PRECIPITATES—Even
if water loss could occur, enzyme inhibitors would neutralize the results. The problem
here is that a powerfully concentrated combination of chemicalized "primitive
water" would be needed to produce the materials of life,—but those very
chemicals would inhibit and quickly destroy the chemical compounds and enzymes formed.
"It is clear that
enzymes were not present in the primordial soup. Even if they were formed, they would not
have lasted long since the primeval soup was by definition a conglomeration of nearly
every conceivable chemical substance. There would have been innumerable enzyme inhibitors
present to inhibit an enzyme as soon as it appeared. Thus, such molecules could not have
formed; however, even with the assumption that they had formed, they could not have
remained."—David and Kenneth Rodabaugh, Creation Research Society Quarterly,
December 1990, p. 107.
Even if they could survive the other
problems, many organic products formed in the ocean would be removed and rendered inactive
as precipitates. For example, fatty acids would combine with magnesium or calcium; and
arginine (an amino acid), chlorophyll and porphyrins would be absorbed by clays.
Many of the chemicals would react with
other chemicals, to form non-biologically useful products. Sugars and amino acids, for
example, are chemically incompatible when brought together.. Sugars and amino acids, for
example, are chemically incompatible when brought together.
The chemical compounds within living
creatures were meant to be inside them, and not outside. Outside, those compounds are
quickly destroyed, if they do not first destroy one another.
CHEMICAL COMPOUNDS AND FLUID
CONDENSATION—In addition to synthesis problems, there are also condensation
problems. Fats, sugars, and nucleic acids can come from the proteins only by very
careful removal of fluid, amid other equally complicated activities conducted by the
laboratory technicians. Those experts spent years in college learning how to do this, yet
it is expected that the sea water earlier did it by itself, using native ingenuity.
Without water loss, proteins cannot form
in water.
"One well-known
problem in the formation of polymerized proteins in water is that water loss is necessary
for this process. Living organisms solve this problem with the presence of enzymes and the
molecule ATP. It is clear the enzymes were not present in the primordial soup."—David
and Kenneth Rodabaugh, Creation Research Society Quarterly, December 1990, p. 107.
CHEMICAL COMPOUNDS AND WATER—So most of the
chemicals needed by life could not arise in a watery environment, such as sea water. In
fact, the lab technicians do their work with fluids other than water! They do not
use sea water or even regular water to make dead amino acids. (That which they synthesize
is always dead; it never has life in it.)
"Beneath the surface
of the water there would not be enough energy to activate further chemical reactions;
water in any case inhibits the growth of more complex molecules."—
*Francis
Hitching, The Neck of the Giraffe (1982), p. 65.
CHEMICAL COMPOUNDS AND ENERGY—And
then there is the problem of `an energy source. Scientists know that there had to be some
form of energy to work the chemical transformations. They generally think it would have
had to have been a bolt of lightning, since there were no wall outlets back in the
beginning to plug electrical cords into. But anything struck by lightning is not
enlivened, but killed!
"[Arrhenius] contends
that if actual lightning struck rather than the fairly mild [electrical] discharges used
by Miller [in making the first synthetic amino acids], any organics that happened to be
present could not have survived."—*Report in Science News, December 1, 1973,
p. 340.
CHEMICAL COMPOUNDS AND OXYGEN—As we
consider all the conditions necessary for a primitive environment in which amino acids and
other biological chemicals could form, we encounter many problems. One of these is the
atmosphere. It is a well-known fact among biochemists that the chemicals of life will
decompose if oxygen is in the air.
"First of all, we saw
that the present atmosphere, with its ozone screen and highly oxidizing conditions, is not
a suitable guide for gas-phase simulation experiments."— *A.L Oparin, Life:
Its Nature, Origin and Development, p. 118.
Living plants and animals only have
certain proportions of the 92 elements within their bodies. These elements are arranged in
special chemical compounds. Chemists say they have been "reduced." When these
particular chemicals are left in the open air, they decompose, or, as the chemists say,
they "oxidize." (A similar process occurs when iron is left in a bucket of
water; it rusts.)
In the presence of oxygen, these
chemicals leave the reduced (or chemical combination) state, and break down to individual
chemicals again. A similar example would be this: Paper will burn in the presence of
oxygen and change back into its member chemicals. Without oxygen, that chemical change
cannot occur.. A similar example would be this: Paper will burn in the presence of oxygen
and change back into its member chemicals. Without oxygen, that chemical change cannot
occur.
"The synthesis of
compounds of biological interest takes place only under reducing conditions [that is, with
no free oxygen in the atmosphere]. "—*Stanley L. Miller and Leslie E. Orgel
(1974), p. 33.
"With oxygen in the air, the first amino acid would
never have gotten started; without oxygen, it would have been wiped out by cosmic
rays."— *Francis Hitching, The Neck of the Giraffe (1982), p. 65.
Later in this chapter, as well as in the chapter appendix,
we go into greater depth on atmospheric problems associated with the primitive
environment.
For additional information
see quotation supplement, "4 - Fighting it out over Early Atmosphere, "
at the end of this chapter.
CHEMICAL COMPOUNDS AND SUPPLY—There
simply would not be enough other chemicals available to accomplish the needed task.
Since most biochemicals contain nitrogen,
it has been discovered by Gish, a biochemist, that there never has been enough
concentration of nitrogen in the air and water for amino acids to form by themselves.
It does not occur naturally in rich enough concentrations.
Similar studies have been made on the
availability of phosphorus by *Bernal. There would not have been enough
phosphorus available for the many chemical combinations needed. Phosphorus is needed
for DNA and other high-energy compounds. But phosphorus concentrations are too low.
Even worse news: *Carl Sagan found that adenosine
triphosphate (high energy phosphate) could not possibly form under the prebiological
conditions.
CHEMICAL COMPOUNDS AND RICH MIXTURES—Since
such a rich mixture of chemicals would have had to be required for the alleged formation
of the first living molecule, there ought to be places in the world where such rich
mixtures are found today, but they do not exist.
"If there ever was a
primitive soup, then we would expect to find at least somewhere on this planet either
massive sediments containing enormous amounts of the various nitrogenous organic
compounds, amino acids, purines, pyrimidines, and the like, or alternatively in much
metamorphosed sediments we should find vast amounts of nitrogenous cokes. . In fact, no
such materials have been found anywhere on earth . . There is, in other words, pretty good
negative evidence that there never was a primitive organic soup on this planet that could
have lasted but a brief moment."—*J. Brooks and *G. Shaw, Origins and
Development of Living Systems (1973), p. 360.

4 — PROTEIN AND OTHER SUBSTANCES
PROTEIN SYNTHESIS—Protein is a
basic Constituent of all life forms. It is composed of amino acids. There are 20 essential
amino acids, none of which can produce the others. How were these made? How could they
make themselves? First, let us examine the simplest of them: glycine.
*Hull figured out that, due to
inadequate chemicals and reaction problems, even glycine could not form by chance.
There was only a 10-27 (minus 27) concentration of the materials needed to make
it. If one glycine molecule was formed, it would have to hunt through 1029
other molecules in the ocean before finding another glycine to link up with! This would be
equivalent to finding one person in a crowd that is 100,000,000,000,000,000,000 times
larger than all the people on earth!
But what about the other nineteen amino
acids? Checking out the others, *Hull found that it was even less possible for them to
form. After careful research, the scientist discovered that as the complexity of
each molecule increased, the possibility of its forming decreased. The concentration
needed for glucose, for example, would be 10-134. That is an extremely high
improbability!
"The conclusion from
these arguments presents the most serious obstacle, if indeed it is not fatal, to the
theory of spontaneous generation. First, thermodynamic calculations predict vanishingly
small concentrations of even the simplest organic compounds. Secondly, the reactions that
are invoked to synthesize such compounds are seen to be much more effective in decomposing
them."—*D. Hull, "Thermodynamics and Kinetics of Spontaneous Generation,
" in Nature, 186 (1960), pp. 693—694.
"In other words, the theoretical
chances of getting through even this first and relatively easy stage [getting amino acids]
in the evolution of life are forbidding."—*Francis Hitching, The Neck
of the
Giraffe (1982), p. 65.
But there is still more:
PROTEINS AND HYDROLYSIS— Even
if protein had been made by chance from nearby chemicals in the ocean, the water in the
primitive oceans would have hydrolyzed the protein. The chemicals that had combined to
make protein, would immediately reconnect with other nearby chemicals in the ocean water,
and self-destruct the protein! The tendency would be for the chemicals to form more
simple organic molecules, such as aldehydes and amines, than the more complex amino acids.
These aldehydes and amines would automatically react with the amino acids, making them
unusable for protein construction. A research team at Bar-Ilan University in Israel, said that
this complication would make the successful making of just one protein totally impossible,
mathematically. It would be 1 chance in 10157. They concluded
that no proteins were ever produced by chance on this earth.
PROTEINS AND SPONTANEOUS
DISSOLUTION—Evolutionists bank on the fact that, somehow, somewhere, in some
way—a small bit of inorganic matter formed some amino acids. Yet even if such an
impossible event could have happened—it would rapidly have disintegrated away!
"In the vast majority
of processes in which we are interested, the point of equilibrium lies far over toward the
side of dissolution. That is to say, spontaneous dissolution [automatic self—destruct
process] is much more probable, and hence proceeds much more rapidly, than spontaneous
synthesis [accidental put—together process] . . The situation we must face is that of
patient Penelope waiting for Odysseus, yet much worse: each night she undid the weaving of
the proceeding day, but here a night could readily undo the work of a year or a century."—*G.
Wald, "The Origin of Life," in The Physics and Chemistry of Life (1955), p. 17.
Automatic dissolution is always easier
than accidental once-in-a-thousand-lifetimes putting-together.
Regarding this massive obstacle to the initial formation of life,
*Wald says it is "the most stubborn problem that confronts us." (Ibid.) Randy
Wysong, in his excellent book, The Creation—Evolution Controversy, provides us
with a clarification of what is involved in this immense hurdle to the initial formation
of life:
"It is conceivable
that wind might blow a pile of toothpicks dumped from a picnic table into an arrangement
resembling a model airplane. Given enough time, it could happen. But if that freak event
does happen, would it remain, if still subject to time and gale winds? Would it even
complexify [later become more complex]? Isn't time not only the creator, but more
efficiently the enemy of the freak event? Will time not surely destroy the order
fortuitously created?"—R.L. Wysong, The Creation-Evolution Controversy, p.
141.
FATTY ACID SYNTHESIS
—Scientists are not able to even theorize how fatty acids
could have originally come into existence.
"No satisfactory
synthesis of fatty acids is at present available. The action of electric discharges on
methane and water gives fairly good yields of acetic and propionic acids, but only small
yields of the higher fatty acids. Furthermore, the small quantities of higher fatty acids
that are found are highly branched."—*S. Miller, and *L. Orgel, The Origins
of Life on the Earth (1974), p. 98 .
OTHER SYNTHESES—There is more
to a living being than merely chemical compounds, proteins, and fatty acids.
There are also enzymes, which
scientists in laboratories do not know how to produce. Yet there are thousands of enzymes
in a typical animal!
Then there are the massive DNA and other coding
problems. Has any scientist ever synthesized a new animal code? No, he would have no
idea how to successfully accomplish the task. The emphasis here is on
"successful." If he could interject a new code, it would only damage the
organism. The list of necessities goes on and on. But what about life itself? One
minute after it dies, an animal still has all its chemicals, proteins, fatty acids,
enzymes, codes, and all the rest. But it no longer has life. Scientists cannot produce
life; why then should they expect rocks and seawater to have that ability?
5 — THE PRIMITIVE ATMOSPHERE
ATMOSPHERE WITHOUT OXYGEN—Could
a non-oxygen atmosphere ever have existed on Planet Earth? It surely seems like an
impossibility, yet evolutionary theorists have decided that the primitive environment had
to have a "reducing atmosphere," that is, one without any oxygen. Now, the
theorists do not really want such a situation, but they know that it would be totally
impossible for the chemical compounds needed for life to be produced outside in the open
air. If oxygen was present, amino acids, etc., could not have been formed. So, in
desperation, they have decided that at some earlier time in earth's history, there was no
oxygen in the air! And then later it got it somehow!
"At that time, the
'free' production of organic matter by ultraviolet light was effectively turned off and a
premium was placed on alternative energy utilization mechanisms. This was a major
evolutionary crisis. I find it remarkable that any organism survived it."—*Carl
Sagan, The Origins, p. 253.
But there is a special reason why they
would prefer to avoid a reducing atmosphere: There is no evidence anywhere in nature
that our planet ever had a non-oxygen atmosphere! And there is no theory that can explain
how it could earlier have had a reducing atmosphere—which later transformed itself
into an oxidizing one! As *Urey himself admitted, a non-oxygen atmosphere is just an
assumption—a flight of imagination—in an effort to accommodate the theory.
"This problem
practically disappears if Oparin's assumptions in regard to the early reducing character
of the atmosphere are adopted."—*Harold Urey, "On the Early Chemical
History of the Earth and the Origin of Life, " in Proceedings of the National Academy
of Science, 38 (1952), p. 352.
*Stanley Miller was one of the pioneers in
laboratory synthesis of non-living amino acids in bottles with a non-oxygen (reducing)
atmosphere. (He was afterward hailed by the press as having "created life."
Miller later said this:
"These ideas are of
course speculation, for we do not know that the earth had a reducing atmosphere when it
was formed."—*Stanley L. Miller, "Production of some Organic Compounds
under possible Primitive Conditions, " in Journal of the American Chemical Society,
77 (1955), p. 2351.
A "reducing atmosphere"
could have had carbon dioxide, methane, hydrogen, ammonia, nitrogen, and water. An
oxidizing atmosphere, such as now exists, would have carbon dioxide, water,
nitrogen, and oxygen.
But a reducing atmosphere could not have
existed earlier on our planet, and especially not when life was supposed to spontaneously
generate. Here are some of the reasons against a reducing atmosphere:
(1) Oxidized Iron. Early
rocks have partly or totally oxidized iron in them (ferric oxide). Oxidized rocks
existed at the time when spontaneous generation is supposed to have taken place. That
proves that the atmosphere had oxygen at that time.. Early rocks have partly or totally
oxidized iron in them (ferric oxide). Oxidized rocks existed at the time when
spontaneous generation is supposed to have taken place. That proves that the
atmosphere had oxygen at that time.
(2) Water means Oxygen. You will
notice, above, that a reducing atmosphere would have had water in it in order for life to
form. There would have had to be water in the atmosphere if there was water on the
earth! Water vapor would continually be arising from the oceans. In the air, that
atmospheric water would be split into hydrogen and oxygen. If our planet once had no
oxygen, it could not have had water either!. You will notice, above, that a reducing
atmosphere would have had water in it in order for life to form. There would have had
to be water in the atmosphere if there was water on the earth! Water vapor would
continually be arising from the oceans. In the air, that atmospheric water would be split
into hydrogen and oxygen. If our planet once had no oxygen, it could not have had water
either!
Referring to that fact, one scientist said
it would be very unlikely for a reducing atmosphere to have existed when biopoiesis
(spontaneous generation of life from non-life) is supposed to have occurred.
"Appreciable oxygen
concentrations might have evolved in the earth's atmosphere before the evolution of
widespread photosynthesizing (oxygen producing) organisms. It does not seem that early
evolution could have proceeded in such an atmosphere."—"R.T.Brinkman,
"Dissociation of Water Vapor and Evolution of Oxygen in the Terrestrial Atmosphere,
" in Journal of Geophysical Research, 74 (1969), p. 5366.
(3) No Life without it. Did
you catch another point in the above quotation? It would have been impossible for
living things to survive, much less evolve, in a reducing atmosphere! How long would
animals live without oxygen to breath? How long would plants live without carbon dioxide?
Without it, they could not make chlorophyll. When plants take in carbon dioxide, they give
out oxygen. But a reducing atmosphere has neither oxygen nor carbon dioxide! Therefore
no plants could either live or be available for food.
(4) Deadly Peroxides. In
addition, a reduction atmosphere could form, through the photolysis of water, into
peroxides, which are deadly to living creatures.
"The hypothesis of an
early methane—ammonia atmosphere is found to be without solid foundation and indeed
is contradicted."—*P. Abelson, "Some Aspects of Paleobiochemistry,
" in Annals of the New York Academy of Science, 69 (1957), p. 275.
(5) No Ozone Layer. Scientists
know that spontaneous generation of life from non-life could not have occurred in the
presence of oxygen, but if there was no oxygen in the atmosphere, there would be no ozone
there either. Without the ozone layer, ultraviolet light would destroy whatever life was
formed.
(6) Ultraviolet Light. Ironically, it
could do more: Just as oxygen in the air would destroy the chemicals of life,
ultraviolet light beaming in through a sky unshielded by ozone would do the same!.
Ironically, it could do more: Just as oxygen in the air would destroy the
chemicals of life, ultraviolet light beaming in through a sky unshielded by ozone would do
the same!
Recent studies of the ozone layer have
revealed that, without it, most living organisms now on our planet would die within an
hour, and many within a second or two!
(7) Not With or Without. Evolutionists
are locked into a situation here that they cannot escape from. Spontaneous generation
could not occur with oxygen—or without it!
With
it there would be rapid oxidation of life chemical compounds and amino acids into separate
chemicals; without it there would be deadly ultraviolet light destroying both the life
chemicals and the life formed from them.
Either way, amino acids would not have
formed, or would quickly break down back into chemicals. On this point alone, it would be
impossible for life to originally have formed out of non-life on Planet Earth.
FORMULA FOR THE PRIMITIVE ATMOSPHERE—The
present atmosphere—the air which we breathe—is composed of carbon dioxide
(C02), nitrogen (N2), oxygen (O2), and water (H20).
The generally postulated primitive
atmosphere would have had to have been composed of almost totally different chemicals: methane
(CH4), carbon monoxide (CO), carbon dioxide (CO2), ammonia (NH2),
nitrogen (N2), hydrogen (H2), and water (H20).
INSTANT ATMOSPHERIC CHANGE!—As
you might imagine, all this bad news brought evolutionary origins to something of a
crisis; especially the problem about the atmosphere.
The response on the part of
intransigent evolutionists was to come up with the really wild theory that at the very
instant when life was created on earth,—at that instant it just so happened that the
entire world changed its atmosphere! It dramatically shifted suddenly from reducing to
oxidizing! That piece of amino acid which had made
itself in the restless oceans, was now able to make itself into part of a protein. (Forget
about the fact that the Law of Mass Action would quickly destroy the amino acid before it
could form protein; forget about the fact that the very presence of oceans made the
atmosphere non-reducing before life began.)
But this possibility collapsed when a
"University of Chicago study found that the plants could not suddenly have made all
that oxygen,—and it had nowhere else to come from! If all the plants NOW on earth
were suddenly formed on Day One of living things on our planet, it would still take
them 5,000 years to produce as much oxygen as we now have!
However, the plants were not there at that
time, and whatever plants might have been there would all have died soon after, since they
themselves need oxygen for their own cellular respiration.
In order to avoid the problem of mass
action degradation of amino acids formed in sea water, someone else suggested that the
amino acids were made in dry clays and rocks. But in that environment either the oxygen
or ultraviolet light would immediately destroy those amino acids.
UNUSUAL CHEMICALS—Men began to
beat their brains against the wall, trying to figure out a way for those amino acids to
form by themselves in the primitive environment.
*Sidney Fox suggested that the amino acids
were made on the edges of volcanoes, *Melvin Calvin decided that dicyanimide
(a compound not naturally occurring in nature) did the job, and *Shramm declared that phosphorus
pentoxide in a jar of ether ether did it! Another research worker came up with an
even more deadly solution: hydrogen cyanide—as the environment in which
all the amino acids made themselves.
But again tragedy struck: It was
discovered that the volcanic heat would ruin the amino acids as soon as they were
formed. Phosphorus pentoxide is a novel compound that could not possibly be
found in earth's primitive atmosphere. The hydrogen cyanide would require an
atmosphere of ammonia, which geological evidence shows never existed in our
atmosphere. Dicyanimide would not work, because the original mixture in
which the first amino acids were made had to have a more alkaline pH.
But on it still goes, one conjecture
after another; always searching for the magic mixture and fairyland environment needed to
make life out of nothing.
"Every time I write a paper on the
origin of life, I determine I will never write another one, because there is too
much speculation running after too few facts."—*Francis Crick, Life Itself
(1981), p. 153. (Crick received a Nobel Prize for discovering the structure of DNA.)
6 — THE LABORATORY EXPERIMENTS
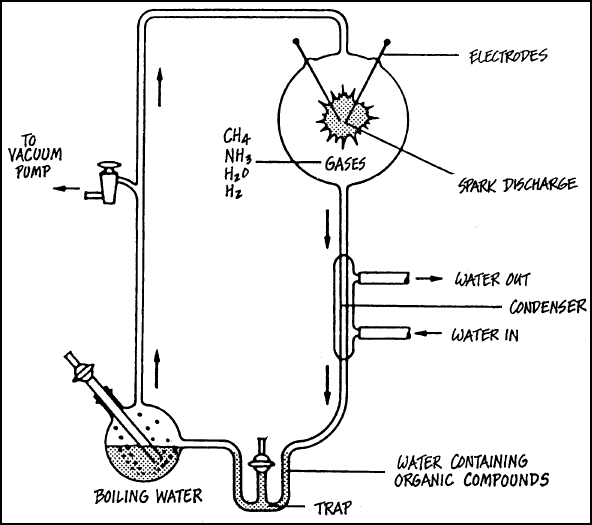
THE MILLER EXPERIMENT—It was
*Stanley Miller in 1953 who first produced amino acids from chemicals. We want to know how
he did it, for THAT is the way the so-called "primitive environment" would have
had to do it by merest chance:
The laboratory apparatus he used to
accomplish this consisted of two confluently interconnected, chemical flasks (or bottles),
arranged one above the other. The lower flask was heated and contained boiling water. The
upper flask contained a mixture of gases including ammonia, methane, hydrogen and water
vapor. (The upper flask had the presumed "primitive atmosphere," since it was
known that if oxygen was present, the experiment would be a failure.)
First, he boiled a mixture of water,
methane, ammonia, and hydrogen gases in the upper bottle, while a small electric spark
continually played over them all. (that was supposed to be equivalent to a gigantic
lightning bolt in the primitive environment which might strike the spot once every so many
years, instantly destroying everything it touched.) The lower bottle of water was kept
boiling in order to keep the mixture in the upper bottle stirred up and
circulating. (the "primitive ocean" must have been pretty hot!) There was a trap
in the bottom of the glass apparatus to catch any soluble organic products, so they would
not be broken down after formation by the spark (chemists knew that the Law of
Mass Action would almost immediately have destroyed the amino acids which were formed,
without a trap to catch them quickly. (The "primitive ocean" must have had
similar bottle traps in it.)
After a week of this, the fluid in the
traps were chemically analyzed—and were found to have microscopic traces of a few L
and D (right- and left-handed) nitrogen-containing compounds—"amino acids,"
they called them—which had been formed. (Of course, if both L and D amino acids were
formed by chemical action—as they always are when formed outside of living
cells—it would be impossible for the amino acid which formed to be usable for life
purposes.)
Newspapers around the world heralded the
news: "Life has been created!" But no life had been created, just a few
biochemical compounds. Remember that neither nitrogen compounds nor amino acids are, of
themselves, living things. Just because they are in living things, does not make them
living things.
In summary then, *Stanley Miller's
experiment was one of the early origin-of-life attempts. It used a reducing atmosphere
(with no oxygen in it). A significant part of his experiment was a "cold trap."
This was a glass cup at the bottom of the tubing which caught the products of the weeklong
water-chemical-spark activity. The purpose of the trap was to keep the reaction going in
the right direction. If it had not been there, the simple amino acids would have been
destroyed faster than they could be made!
"'This is the
primitive atmosphere,' said Stanley Miller, the chemistry professor at the University of
California at San Diego, as he pointed to the transparent mixture of gases inside the
globe. `And this represents the primitive ocean,' he said, indicating a pool of water in
the bottom of his apparatus."—*Rick Gore, "Awesome Worlds Within a Cell,
" National Geographic Society, September 1976, p. 390.
What does that carefully contrived
experiment have to say about the possibility of a man doing it out in the field with the
random chemicals found in dirt, and the three things that would ruin the outcome:
death-dealing lightning, oxidizing oxygen, and hydrolyzing water? And where would the
continual spark come from in nature? There is none available, other than from lightning or
molten lava. Lightning does not create life; it destroys it! And fiery volcanic magma is
no better.
"In 1953 two
scientists, Harold Urey and Stanley Miller, performed one of the mast striking experiments
of the twentieth century. In a pressure vessel they mixed simple molecules simulating the
primordial atmosphere of Earth. Then they zapped the vessel with electricity to simulate
lightning bolts . .
"The notion that biological
substances could arise from a purely natural process made scientists cheer and gave the
clergy chills. But on reflection, less had happened than met the eye. Though the goo in
Urey and Miller's beaker contained ingredients used by life, it did not come to life. It
was just interesting goo. Now as then, nobody has any idea what makes chemicals start
living. The origin of life is perhaps the leading unknown of contemporary science."—*G.
Easterbrook, "Are We Alone?" in The Atlantis, 262(2):32 (1988).
What does that complicated lab experiment
have to say about the possibility of nature doing it by accident—without the help of
man? Outdoors, it could not be done without his help, or with it.
"What we ask is to
synthesize organic molecules without such a machine. I believe this to be the most
stubborn problem that confronts us the weakest link at present in our argument. I do not
think it by any means disastrous, but it calls for phenomena and faces, some of which are
as yet only partly understood and some probably still to be discovered."—
*G.
Wald, "The Origin of Life," in the Physics and Chemistry of Life
(1955),
p. 9.
LABORATORY APPARATUS FOR THE MILLER EXPERIMENT
A few non-living specs of amino acids were
produced by "Stanley Miller in 1953, using the following laboratory equipment. The
resultant amino acids had been made in an equal amount of left- and right-handed (L and D)
forms, so they were useless to already-living tissue, much less in making it!
Notice what it took to produce such
pathetic results: A vacuum pump to continually circulate the vapors, special tubing sealed
away from the outside world, special distilled water inlets and outlets, electric element
producing 212° F. [100°C.] water temperature, electrical contacts to make a continuous,
very low—amperage spark, and a trap arrangement to immediately siphon off nitrogenous
products before they were destroyed in the boiling water and resultant vapors.
Where in the world could you find such a
"primitive environment"? Even if it could exist, non-living L and D amino acids
would be all that would result.
The test tube attempts to "create
life" have only resulted in dismal failure.
"In 1953, at the
University of Chicago, Stanley L. Miller and Harold C. Urey mixed ammonia, water
vapor, hydrogen and methane to simulate Earth's early atmosphere, then crackled
lightning—like electrical sparks through it . .
"Unfortunately, as Margolis admits,
‘no cell has yet crawled out of a test tube,' and thousands of similar experiments
have produced gooey organic tars, but no recognizable life. Decades of persistent failure
to 'create life' by the 'spark in the soup' method (or to find such productions in nature)
have caused some researchers to seek other approaches to the great enigma. [Panspermia
theories are then discussed.]"—*Richard Milner, Encyclopedia of Evolution
(1990), p. 274.
NOT THE RIGHT AMINO ACIDS—Not only do the
Miller-type experiments not produce the proper "handedness" of amino acids
(left-handed amino acids only, instead of both-handed ones), but that type of
experiment—which has been repeated many times in the decades since Miller first did
it—consistently does not produce just the crucial amino acids needed for life. Out of
the hundreds of possible combinations, there are 20 essential amino acids, and laboratory
synthesis of amino acids produce only a few of them—along with a lot of non-essential
or even useless ones.
"In considering
Miller's 1953 experiment and subsequent experiments where amino acids were formed through
applying heat to elements alleged to be in the primordial atmosphere, the author mentions:
(1) that these amino acids were racemic (both D and L forms) and thus proteins
formed from these would not support life; (2) the majority of amino acids [formed by
laboratory synthesis] do not belong to the 20 amino acids that occur in natural protein
molecules."—David and Kenneth Rodabaugh, "Book Review," Creation
Research Society Quarterly, December 1990, p. 107.
THE OPARIN EXPERIMENT—Somewhat
before *Miller, *A.I. Oparin, a Russian chemist, attempted something similar. He had a
"coacervate hypothesis" which he believed would eventually produce
living cells. "Coacervates" are like fat droplets in a bowl of soup. He
carefully kept all oxygen away from the soup and the bowl, and he hoped that, given enough
time, they would join together and, somehow, life would enter into them!
Oparin discovered that coacervates are
highly unstable. The thin outer film breaks easily. Collections of them break apart
easily. They quickly unite with other nearby molecules. There would be no selectivity as
to molecules absorbed. Harmful as well as helpful ones would be as easily absorbed. No
reputable chemist today considers Oparin's theory to be of any value.
THE FOX EXPERIMENTS—After
Miller's experiment, *Sydney Fox in 1960 worked out a different arrangement, but he
began his with amino acids already formed! He claims that his method is how it was done in
the primitive environment. This should have been good news for the evolutionary world,
but when we learn his complicated procedure, we can understand why few scientists have any
faith in the possibility that the Fox procedure was done by chance in the ocean, near a
volcano, or in a mud puddle.
Here is how nature, armed with time and
chance, is expected to have produced that first dead amino acid:
"Typical
panpolymerization: Ten grams of L-glutamic acid [a left amino acid] was heated at 175-180
C. [347°356°F.] until molten (about 30 minutes), after which period it had been largely
converted to lactum. At this time, 10 g. [352 av. oz.) of DL-aspartic acid and 5 g. [.176
av. oz.] of the mixture of the sixteen basic and neutral (BN) amino acids were added. The
solution was then maintained at 170° + or -2° under an atmosphere of nitrogen for
varying periods of time. Within a period of a few hours considerable gas had been evolved,
and the color of the liquid changed to amber. The vitreous mixture was rubbed vigorously
with 75 ml. [4.575 cu. in.] of water, which converted it to a yellow-brown granular
precipitate. After overnight standing, the solid was separated by filtration. This was
washed with 50 ml. [3.05 cu. in.] of ethanol, and as substance S dialytically washed in
moving Multidialysers in water for 4 days, the water being changed thrice daily. (The term
dialytic washing indicates dialytic treatment of a suspension.) In some preparations, the
solid was dissolved completely in sodium bicarbonate solution and then dialyzed. The
dialysis sacs were made of cellulose tubing, 27/32 in., to contain 50 ml. [3.05 cu. in.].
The nondiffusible material was ninhydrin-negative before the fourth day. The non-aqueous
contents of the dialysis sac were mainly solid A and a soluble fraction B recovered as
solid by concentration in a vacuum dissicator. The mother liquor of S was also dialyzed
for 4 days, and then dried to give additional solid C."—*S. W. Fox and *K.
Harada in Journal of
the American Chemical Society, 82 (1980). p. 3745.
There may be some words and chemical
processes in the above description with which you are unfamiliar, but it is clear that what
those men did required an exceedingly complex procedure, superior intelligence, high-level
training, a well-equipped laboratory, and many, many days of hard work carried out
according to an elaborate plan.
We commend *Sydney Fox and his associates
for their remarkable intelligence and excellent lab equipment, and the university
scientists who trained them so well to perform such experiments, but we can make no such
commendation of sand, gravel, and seawater which is supposed to have done the same thing
by itself.
Fox began with a quantity of left-only (no right) amino
acids and made sure no sugars were present, since they would nullify each other. Then he
underwent a lot of tedious work that requires a high degree of intelligence, careful
planning, and many adjustments with pH, temperature, cooking time, etc., as he proceeded
with a staff of assistants to help him succeed:
Fox is modest about his abilities, for
he says that random events, in a broad sea or on the slopes of a volcano, could have done
it as easily. But HE began with pure, left-handed amino acids; he did not begin with
pebbles, mud, and water.
Fox then heated the amino acids for 10
hours 150-1800C [302-3560F]. He said that this originally happened
for 10 hours in a dry spot on the edge of an ancient volcano, so 150-1800C
[302-3560F] for 10 hours with a total lack of moisture would be necessary.
Where would you find such conditions in
nature? *Stanley Miller, who first synthesized amino acids in a laboratory later stated
that his own experiment could not possibly have been done by chance outside of a modern
laboratory. Others agree.
"The degree to which experimental
conditions actually simulate primitive earth conditions is very often the subject of
considerable controversy among workers in the field [of biochemistry]."—*A.I.
Oparin, Life: Its Nature, Origin, and Development, p. 33.
The tiniest living organism (a bacteria)
has many specific functional parts, with each part dependent on the other. There is a
purpose to everything within it. Can all that be made in non-oxygen containers, cold
traps, 10 hours of hot, dry heat, or subjection to a week of sparking?
"Such experiments are no more than
exercises in organic chemistry."—*P. Mora, "The Folly of
Probability, "in Origins of Prebiological Systems and their Molecular Matrices, Ed.
S. W. Fox (1965), p. 41.
Three key ingredients are (1) proper
chemicals in exacting amounts, (2) a continuous energy source (such as a continuous
spark), and (3) quick-dry apparatus. As soon as the amino acids are made, they must be
immediately dried out. (Living tissue never contains dried-out amino acids or comes from
it.) *Fox tells us the reaction must be "hot
and dry" (Origins of Prebiological Systems and their Molecular Matrices, p. 378).
"To keep a reaction going according to
the law of mass action, there must be a continuous supply of energy and of selected matter
(molecules) and a continuous process of elimination of the reaction products.
"—*P.
More, "The Folly of Probability, " in Origins of Prebiological systems and their
Molecular Matrices, Ed. S. W. Fox (1965), p. 43.
And there is a fourth key ingredient:
careful organization with specific purposes by intelligent, highly-trained minds doing the
work on the chemicals. No one tosses the chemicals into a pan in the laboratory, walks
off, and hopes it will all produce amino acids by itself.. No one tosses the chemicals
into a pan in the laboratory, walks off, and hopes it will all produce amino acids by
itself.
A living organism is not just dried out
ocean soup. It is highly integrated, complex, and purposive. It also has life, which no
man can produce.
7 — CONCLUSION
LIFE NICHE LIMITS
7 — CONCLUSION
LIFE NICHE LIMITS—A new way to
consider an old fact has surfaced in recent years. It is called "life niches"
or "niche spaces. " Consider for a moment a bacterium.
Certain conditions are necessary so it can live. Scientists tell us to imagine that
the bacterium is located inside a cube-shaped box. The height of the box is the range of
one condition necessary for its life; the depth indicates the span of another
specification, and the width indicates a third life-requirement range. If one of the three
ranges narrows too much, the bacterium will die. For example, if its heart stops beating.
Certain things must occur, keep
occurring, and occur right—in order for an organism to keep living. When one of them
narrows too much, it dies.
Now, let us turn to reality. The
conditions of life are far more than three things. Instead of the scientist's "life
niche box," let us visualize a vertical bar graph. Each bar measures one range of
tolerances, within which a human being can continue to live. How many bars are there?
BILLIONS of them! One set of hundreds of thousands of bars measures factors in the bone
marrow that must be exactly right in order for us to produce blood and remain alive.
Another set of hundreds of thousands—perhaps millions—of factors concern
conditions in the pituitary. And on it goes. How long is the entire bar graph of
required life specifications needed for you and me to keep living? ft probably reaches to
the moon and back.
How long is the bar graph for that
bacterium? Probably three-quarters of the distance to the moon, all of it filled—side
by side—with bar graphs.
Now for the punch line: Each bar on the
graph had to be in place for that bacterium to first exist
But there is more: From the very
beginning, that bacterium had to have a mate. So the impossible had to occur in a
gender duplicate for every life form made.
And there is still more: That bacterium
had to have its proper food supply immediately. Its food was organic—living,—so
its food had to have its own bar graph of specifications reaching almost to the moon!
In addition, many life forms are
interrelated, forming a dietetic chain. So now we have many different life forms, each
depending on one another, and each with its own lengthy bar graph of needs.
For more details on the
immense complexity of all this, see chapter 11,
COMPLICATED AND INTERRELATED REQUIREMENTS—There are
far more requirements for life to successfully evolve than one might at first think.
Indeed, the more thought we give to the matter, the more we realize that only the ignorant
could conceive of a random self-origination and evolving of living creatures.
1—Symbiotic Relationships. There
are many instances in which quite different life forms rely on one another. Neither
the yucca plant nor the pronuba moth could exist without one another. The fig tree and the
fig gall wasp are another interdependent team. The cow could not digest its food without
certain bacteria in its stomach, and neither could the termite. In the beehive, the queen,
workers, and drones are totally dependent on one another. How could all this have been
initiated? There really is no way it could come about by accident. Those organized and
interrelated patterns had to be there from the start.
2 — Immune Systems. All
animals and some plants have extremely complicated immune systems to protect them. Yet
they had to have those immune patterns——dating back to the beginning. If
not, they would never have survived long enough to develop them. Each immune system can
identify bacteria, viruses, and toxins—and recognize whether each is safe or
harmful. Each system has a complete, complex pattern for organizing a variety of soldiers
to eliminate such problems as soon as possible. In fact, each invasion is indelibly
remembered by the soldiers, so they can better protect the body the next time. The immune
system could not slowly evolve; it had to completely be there to begin with.
3 — Fantastic Technology. There
are technological wonders all through nature that are astounding.
Consider the miniature sonar systems of porpoises and whales, the
frequency-modulated radar (sonar) system of the bat, the aerodynamic capabilities of the
hummingbird, the precise navigational systems of birds and fish. On and on we could go.
Yet that technology was there to begin with.
On the very first day of his existence,
the little hummingbird had to have that long beak to dip sugar water out of the flowers.
The flowers had to be there also. The hummer had to have an extremely fast metabolic
system, able to live on such a powerful solution. It had to have extremely fast wings, in
order to hover over flower after flower, all day long.
4 — Sexual Reproduction.
If animals evolved, as the theory teaches, then those random
accidents, known as "natural selection," had to do amazing things.
It was
necessary, from the very beginning, for both a male and a female of each species to be
there—or that species would quickly perish. Evolutionists cannot explain why
sexual reproduction exists. Evolution could be accomplished so easily without it! Yet
nearly all plants and animals continue on from generation to generation because of it.
Both the male and female of each species had to evolve totally independently of the
other—and yet at each "phase of evolution," the two were matching partners.
This pairing of the species could neither originate nor evolve by evolutionary means.
CONCLUSION—We have viewed the
desolate attempts to figure out a way to produce living tissue, plants, and animals out of
sloshing water and sand. Oddly enough, this desperate research is said to have begun with
*Charles Darwin, but ironically, Darwin only theorized about evolution across species
by natural selection; he never discussed the origins of life.
"Darwin never really
did discuss the origin of species in his On the Origin of
Species."—*David Kitts, "Paleontology and Evolutionary Theory,"
Evolution, Vol. 28, September 1974, p. 488.
Reputable scientists tell us that life
could neither originate nor continue without intelligence being involved.
"Any living thing
possesses an enormous amount of 'intelligence' . . Today, this 'intelligence' is called
'information,' but it is still the same thing . . This 'intelligence' is the
sine qua
non of life. If absent, no living being is imaginable. Where does it come from? This
is a problem which concerns both biologists and philosophers, and, at present, science
seams incapable of solving it."—*Pierre-Paul Grasse, Evolution of Living
Organisms (1977), p. 3.
But evolutionists are hopeful that they
will yet solve the problem. *Carl Sagan, a leading science (and science fiction) writer
says that the people on Mars may help get us straightened out on this matter. Sagan has
been speculating about extraterrestrial life for many years, and is hopeful that Martian
life will convince people once and for all that life has evolved:
"If it turns out that
there is life there as well, then, I would say, it would convince large numbers of people
that the origins of life exist."—*Science News symposium entitled, "Life
on Mars: What Could It Mean?" Vol. 109, June 5 & 12, 1978, pp. 378-379.
For additional
information see supplement, "5 - Searching for Life Elsewhere," at the end of
this chapter.
A FEW OF THE PROBLEMS TO BE
SOLVED. —
1 — SPONTANEOUS GENERATION HAS BEEN SCIENTIFICALLY
DISPROVED
2 — INSTANT SUCCESS WOULD HAVE TO BE NECESSARY FOR
THE LIFE FORM TO SURVIVE
3 — THOUSANDS OF ESSENTIAL BODY PARTS AND THOUSANDS
MORE OF ESSENTIAL CHEMICAL COMPOUNDS WOULD HAVE TO INSTANTLY FORM THEMSELVES
4 — BOTH MALE AND FEMALE FORMS WOULD NEED TO MAKE
THEMSELVES AND NEAR EACH OTHER IN SPACE AND TIME
5 — LAW OF MASS ACTION WOULD IMMEDIATELY DESTROY
CHEMICAL COMPOUNDS
6 — WATER IS NEVER CONCENTRATED ENOUGH TO PRODUCE
LIFE CHEMICALS
7 — THERE IS NO LAB EQUIPMENT OUT IN NATURE
8 — CONDENSATION PROBLEM: WATER MUST BE CAREFULLY
REMOVED FOR FATS, SUGARS, AND NUCLEIC ACIDS TO DERIVE OUT OF PROTEIN
9 — PRECIPITATION PROBLEM: ENZYMES WOULD IMMEDIATELY
BE DESTROYED
10 — MOST LIFE CHEMICALS NOT FOUND IN WATERY
ENVIRONMENT
11 — LIGHTNING BOLTS ONLY DAMAGE OR KILL AND COULD
NOT BE THE ENERGY SOURCE
12 — OXYGEN PROBLEM: LIFE COULD NOT ORIGINATE WHERE
THERE IS OXYGEN
13 — LIFE COULD NOT SURVIVE WITHOUT CONTINUAL OXYGEN
14 — OXYDIZED IRON IS FOUND IN ROCKS EXISTING WHEN
LIFE IS SAID TO HAVE ORIGINATED
15 — LIFE COULD NOT ORIGINATE WITHOUT WATER, BUT
THERE CAN BE NO WATER WITHOUT OXYGEN
16 — A REDUCING ATMOSPHERE WOULD PRODUCE LIFE-KILLING
PEROXIDES
17 — ULTRAVIOLET LIGHT IN REDUCING ATMOSPHERE WOULD
IMMEDIATELY KILL LIFE
18 — WITHOUT OXYGEN THERE WOULD BE NO PROTECTIVE
OZONE LAYER
19 — PROTEINS WOULD IMMEDIATELY HYDROLYZE AND DESTROY
THEMSELVES
20 — THERE WOULD NOT BE ENOUGH CHEMICALS AVAILABLE TO
FORM EVEN THE SIMPLEST PROTEIN
21 — NITROGEN IS IN MOST BIOCHEMICALS, BUT THERE IS
NOT ENOUGH
CONCENTRATED NITROGEN IN NATURE TO FORM LIFE
22 — THERE IS NOT ENOUGH AVAILABLE PHOSPHORUS IN
NATURE EITHER
23 — SCIENCE HAS NO IDEA HOW TO MAKE FATTY ACIDS, OR
HOW THEY COULD MAKE THEMSELVES
24 — THE ATMOSPHERE THROUGHOUT THE WORLD WOULD HAVE
TO INSTANTLY CHANGE FROM NO OXYGEN TO ITS PRESENT OXYGEN-RICH CONTENT
25 — EXTREMELY COMPLICATED CHEMICAL COMBINATIONS NOT
FOUND IN
NON-LIVING MATERIALS EXIST IN LIVING TISSUE
26 — RESIDUE PROBLEM: SINCE SUCH EXTREMELY RICH
CHEMICAL MIXTURES ARE FOUND IN LIVING THINGS, WE SHOULD FIND RESIDUES OF THEM IN NATURE,
BUT THEY DO NOT EXIST
27 — ACCIDENTAL FORMATION OF AMINO ACIDS WOULD
PRODUCE EQUAL AMOUNTS OF LEFT—AND RIGHT—HANDED ONES, BUT ONLY LEFT—HANDED
FORMS EXIST IN ANIMAL LIFE
28 — DISSOLUTION PROBLEM: EVEN IF THE CORRECT
CHEMICALS COULD GATHER TOGETHER, THE NEXT INSTANT THEY WOULD SPONTANEOUSLY DISINTEGRATE,
BY REFORMING WITH OTHER CHEMICALS
29 — IMMEDIATE, COMPLETE DUPLICATION AND REPRODUCTION
OF DNA, PROTEIN, ENZYMES, FATS, CELLS, ETC., WOULD BE NEEDED FOR SURVIVAL
30 — THERE IS NOT THE REMOTEST POSSIBILITY LIFE COULD
ORIGINATE BY ITSELF. THERE IS NOT ENOUGH TIME AND SPACE IN ALL THE UNIVERSE IN ALL
ETERNITY TO PRODUCE OUR PRESENT MYRIAD OF LIVING SPECIES ON EARTH

Trying to find self-originated life on
other planets, is but to ignore the solidly researched fact that it could not originate by
itself right on our own. Scientists who have spent a lifetime trying to figure out the
origins of life on our planet openly state their conclusion:
"An honest man, armed
with all the knowledge available to us now, could only state that in some sense, the
origin of life appears at the moment to be almost a miracle."—*Francis Crick,
Life Itself, Its Origin and Nature (1981), p. 88.
"The present laws of physics... are
insufficient to describe the origin of life. To him this opens the way to teleology, even,
by implication, to creation by an intelligent agent. . If he thinks he has shown
conclusively that life cannot have originated by chance, only two rational alternatives
remain. The first is that it did not arise at all and that all we are studying is an
illusion."—*S. W. Fox, The Origins of Prebiological Systems and Their
Molecular Matrices (1965), pp. 35-55.
A Nobel Prize laureate and a confirmed evolutionist made
this comment:
"All of us who study
the origin of life find that the more we look into it, the more we feel it is too complex
to have evolved anywhere. We all believe as an article of faith that life evolved from
dead matter on this planet. It is just that its complexity is so great, it is hard for us
to imagine that it did."—*Harold C. Urey, quoted in Christian Science
Monitor, January 4, 1962, p. 4.
THE MAGIC FORMULA—The formula for the
evolutionary origin and development of life goes something like this:
NOTHING + TIME + CHANCE = "SIMPLE" CELL +
TIME + CHANCE = MAN
Is this modern science, or is it a fairy
tale? It is an astounding thought that all modern biological, genetic, and geological
science is keyed to such a mythical formulation.
One evolutionist explains in philosophical
rhetoric how it all happened:
"Randomness caught on the wing,
preserved, reproduced . . and thus converted into order, rule, necessity. A
totally blind
process can by definition lead to anything; it can even lead to vision itself."—*Bur,
quoted in *Jacques Monod, Chance and Necessity (1972), p. 98.
That may sound good, but it is neither
true nor scientific. If randomness can produce such living wonders as are all about us,
even human eyesight, than highly-intelligent scientists, working in well-equipped
laboratories, ought to be able to produce eyes, ears, and entirely new species in a few
month's time.
The Great Evolutionary Myth Is that
randomness plus time can do anything; the truth is that randomness, with or without time,
can accomplish almost nothing. And those changes which it does accomplish will quickly be
blotted out 6y the next random action or two
"All the facile
speculations and discussions published during the last ten to fifteen years explaining the
mode of origin of life have been shown to be far too simple-minded and to bear very little
weight. The problem in fact seems as far from solution as it ever was."—*Francis
Hitching, The Neck of the Giraffe (1982), p. 68.
THE EVOLUTIONARY ORIGIN OF LIFE IN A
NUTSHELL—Origin of life by random means is an impossibility of the
impossibilities. After what you have learned in this chapter, it should now be fairly easy
for you to see, in the following evolutionary five-step theoretical program of events,
that it consists of little more than arm-chair guessing combined with Alice in Wonderland
hopefulness:
"Evolution Model for
the Origin of Life on the Earth:
"According to the evolution model,
the story of life on the earth began some five billion years ago and gradually unfolded
through a series of five stages:
"Stage 1. Evolutionists have
imagined that the atmosphere of the early earth was quite different from the
present atmosphere. In contrast to the present oxidizing atmosphere, which contains
21 percent free oxygen (02), 78 percent nitrogen (N2), and 1 percent
of other gases, supposedly the early earth was surrounded by a reducing atmosphere made up
mostly of methane (CH4), ammonia (NH2), hydrogen (H2),
and water vapor (H20).
"Stage 2. Because of
ultraviolet light, electric discharge, and high—energy particle bombardment of
molecules in a reducing atmosphere, stage 2 came about with the formation of small organic
molecules such as sugars, amino acids, and nucleotides.
"Stage 3. Presuming all of
this happened billions of years ago in a reducing atmosphere, then stage 3 is imagined
during which combinations of various small stage 2 molecules resulted in formation of
large polymers such as starches, proteins, and nucleic acids (DNA).
"Stage 4. These large
molecules supposedly joined together into gel—like globs called coacervates or
microspheres. Possibly these coacervates attracted smaller molecules so that new
structures, called proto—cells, might have foamed.
"Stage 5. Evolutionists
believe that, finally at least one of these globs absorbed the right molecules so that
complex molecules could be duplicated within new units called living cells. These first
cells consumed molecules left over from earlier states, but eventually photosynthesis
appeared in cells, in some way, and oxygen was released into the atmosphere. As the
percentage of oxygen in the early atmosphere increased, most of the known forms of life on
the earth today began to appear. Because of the presence of oxygen, these early life forms
destroyed all the molecules from earlier stages, and no more chemical evolution was
possible."—John N. Moors, "Teaching about Origin Questions: Origin of
Life on Earth, " in Creation Research Society Quarterly, June 1985, page 21.
APPLYING MATH TO IT—"Sir
Fred Hoyle, the famous British mathematician and astronomer, teamed up with *Chandra
Wickramasinghe in an analysis of the origin of life and the possibility that it could
possibly have begun by chance.
Hoyle started out on this project as an
evolutionist, and Wickramasinghe as a Buddhist. Hoyle ended up as leaning toward a belief
that God created everything.
They mathematically determined that the
likelihood that a single cell could originate in a primitive environment, given 4.6
billion years in which to do it—was one chance in 1040000!
That is one chance in 1 with 40 thousand zeros after it! Speaking
about these early environment controversies, they said this:
"The tactic is to
argue that although the chance of arriving at the biochemical system of life as we know it
is admitted to be utterly minuscule [extremely small], there is in Nature such an enormous
number of other chemical systems which could also support life—that any old planet
like the Earth would inevitably arrive sooner or later at one or another of them.
"This argument is the eeriest
nonsense, and if it is to be imbibed at all it must be swallowed with a jorum of strong
ale."—*Fred Hoyle and *Chandra Wickramasinghe, Evolution from Space (1981),
p. 28.
Everything would suddenly have to be
there all at once. It would all have to work perfectly, and it would have to split and
divide into new cells immediately, and reproduce offspring quickly. Living forms are too
awesome to relegate to the tender mercies of time and chance. It took special design,
special thinking, special power to make living beings.
And that brings us to our
next chapter: the incredible wonders of DNA and the impossibility of it
accidentally making itself out of chance, gravel, mud, and water.
You have just completed
CHAPTER 9
THE PRIMITIVE
ENVIRONMENT Part 2
APPENDIX - 9






